Joenja results
Discover how Joenja was studied and
results from the clinical trial.

Annie, person living with APDS

How Joenja was studied

Duration
12 weeks

Population
31 participants

Age
12 years and older
Dosing
Each participant took a fixed 70-mg dosage of Joenja or placebo, twice a day
Conditions
The participants, sponsor staff, and trial staff did not know what treatment each participant took. This prevented participants from influencing some of the results

People with APDS taking Joenja saw improvement in 2 important ways:
The size of the most swollen lymph nodes was reduced
- The size of swollen lymph nodes for each person in the trial was studied before and after treatment
- The results showed that lymph nodes shrank more in people taking Joenja vs those receiving placebo
Joenja
(n/N=18/21)
Placebo
(n/N=8/10)
Baseline mean (SD)
3.03 (0.42)
3.05 (0.39)
Change from baseline, LS mean (SE)
-0.27 (0.04)
-0.02 (0.05)
Difference vs placebo (95% CI)
-0.25 (-0.38,-0.12)
Reduction of swollen lymph nodes*†‡
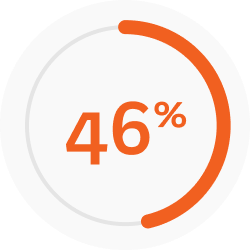
That is a ~8x greater improvement with Joenja vs placebo
*
Reduction computed based on estimates for the adjusted mean changes.
†
The LS mean change from baseline, difference in LS mean change from baseline between Joenja and placebo and its P value, were obtained from an ANCOVA model with treatment, glucocorticoid use, and immunoglobulin replacement therapy at baseline, and baseline measurement as covariates.
‡
Change in index lesion size was measured using the log 10-transformed SPD of the largest lymph nodes (maximum of 6) identified as per the Cheson criteria on CT/MRI.
§
The analysis excluded two patients from each treatment group due to protocol deviations and one Joenja patient having complete resolution of the index lesion identified at baseline.
ANCOVA, analysis of covariance; LS, least squares; SPD, sum of product diameters.
Increase in functioning B cells that was maintained over 12 weeks
- Some participants began the trial with fewer than 48% of their B cells functioning properlyII
- The increase in functional B cells helps restore balance to your immune system¶
- B cells are a type of white blood cell that help the body fight infections
Absolute percentage of naïve B cells over timeII
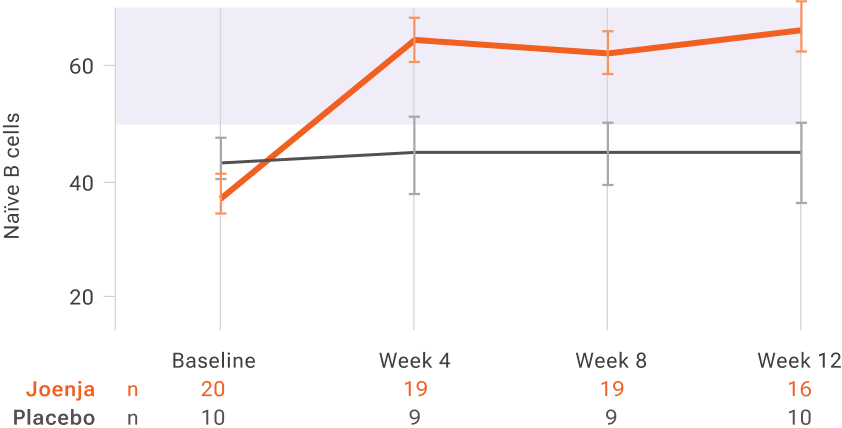
Functional B-cell levels returned to normal range within 4 weeks and remained steady through week 12
II
Normal range for percentage of naïve B cells indicated by shaded bar in graph.
¶
Cell surface markers used to distinguish naïve B cells on flow cytometry were CD19+, CD27-, and CD10-. Baseline is defined as the arithmetic mean of the baseline and day 1 values when both were available, and if either was missing, the existing value was used.
The analysis excluded 2 patients from each treatment group due to protocol deviations, 5 Joenja patients and 3 placebo patients with ≥48% naïve B cells at baseline, 5 Joenja patients with no day 85 measurement, and 1 Joenja patient with no baseline measurement.

Possible side effects of Joenja
If you have concerns or would like to know more about potential side effects, please talk with your doctor about Joenja.
Joenja
21 participants
n (%)
Placebo
10 participants
n (%)

Headache
5
(24%)
2
(20%)

Sinus infection
4
(19%)
0
(0%)

Skin rash
3
(14%)
0
(0%)

Elevated heart rate
2
(10%)
0
(0%)

Diarrhea
2
(10%)
0
(0%)

Fatigue
2
(10%)
1
(10%)

Fever
2
(10%)
0
(0%)
Back pain
2
(10%)
0
(0%)
Neck pain
2
(10%)
0
(0%)

Hair loss
2
(10%)
0
(0%)
Tell your healthcare provider if you are scheduled to receive an immunization (vaccine). Joenja may affect how well a vaccine works.

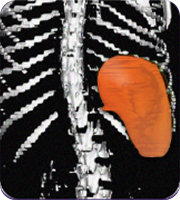
Patients with an enlarged spleen saw an average 27% decrease in the size of their spleen#
The spleen plays an important role in filtering out infections from our blood. When the spleen becomes enlarged, it may not function properly.
Secondary measure: spleen size scan results of actual patient show average improvement for patients taking Joenja
Prior to treatment
491 mL
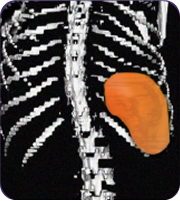
At week 12
314 mL
Actual images of a 29-year-old female’s response of spleen size reduction, representing the average
response in the study. As individual results vary, images may not be representative of all patients.
#
In the PD analysis set, the mean (SD) percentage change from baseline to week 12 in 3-D spleen volume (mm3) was -26.68% (12.137) with Joenja (n=19) and -1.37% (24.238) with placebo (n=9). The ANCOVA model was used with treatment as a fixed effect and log10-transformed baseline as a covariate for index and non-index lesions. The use of both glucocorticoids and IV immunoglobulin at baseline was included as categorical (yes/no) covariates.
This analysis excluded 2 patients in each treatment group. In the Joenja group, 1 patient with a complete index lesion response was excluded, and 3 patients were excluded for no non-index lesion at baseline.
IV, intravenous; PD, pharmacodynamics; SD, standard deviation.

The safety of Joenja continues to be studied
- After the completion of the 12 weeks of the original study, Joenja continued to be studied in 37 of the original 38 patients
- The patients included in this study extension were taking Joenja for an average of 2 years
- Four of these patients had been taking Joenja for more than 5 years
- While the study is ongoing, the data below reflect the results at the first data cutoff period
Most common side effects in study extension
- Upper respiratory tract infection
- Headache
- Fever
- Ear infection
- Weight gain
- COVID-19
Side effects associated with Joenja
- Weight gain
- Joint pain
- High blood sugar
- Neutropenia
Side effects not associated with Joenja
- 32 of the 37 patients experienced side effects
- 78% of side effects experienced in the clinical study were mild and determined not to be related to Joenja
- 49% of side effects were considered moderate
- 27% of side effects were considered severe or medically significant, but not life-threatening
- No life-threatening side effects were seen
- One grade 5 patient with significant baseline comorbidities suffered cardiac arrest resulting in death on day 879; investigator determined that the death was not related to Joenja
APDS, activated PI3K delta syndrome.
Learn more about taking Joenja
→
Learn more about
APDS Assist
→
Right Circle
Left Circle



